Cspec Editor
This page provides tutorials and illustrations on how to use the CSpec Editor UI and its features.
- Introduction to CSpec Editor
- User Management
- Entry Page
- Creating a new specification document
- Detailed view of a specification document
- Reopen a previously released/existing specification document
- Release an approved document
- Type-specify a criteria specification document
Introduction to CSpec Editor
ClinGen Criteria Specification Editor is an application which is designed primarily to allow users with appropriate access and permissions to do the following:
- Create new criteria specification document
- Edit previously existing specification documents
- Reopen, submit specification documents for approval and release approved criteria specification documents
The Editor in general will also allow to
- Track the specification approval process and versioning of these documents
- Transfer content to external applications via ClinGen Data Exchange
The beta version of the Editor is available here https://cspec.genome.network/cspec/ed/svi
User Management
All users must be logged in to the cspec editor to gain access to the documents that are affiliated to their vceps. One can access the login form here - https://cspec.genome.network/cspec/ed/svi. Type in your credentials in the form shown below: 
First time users
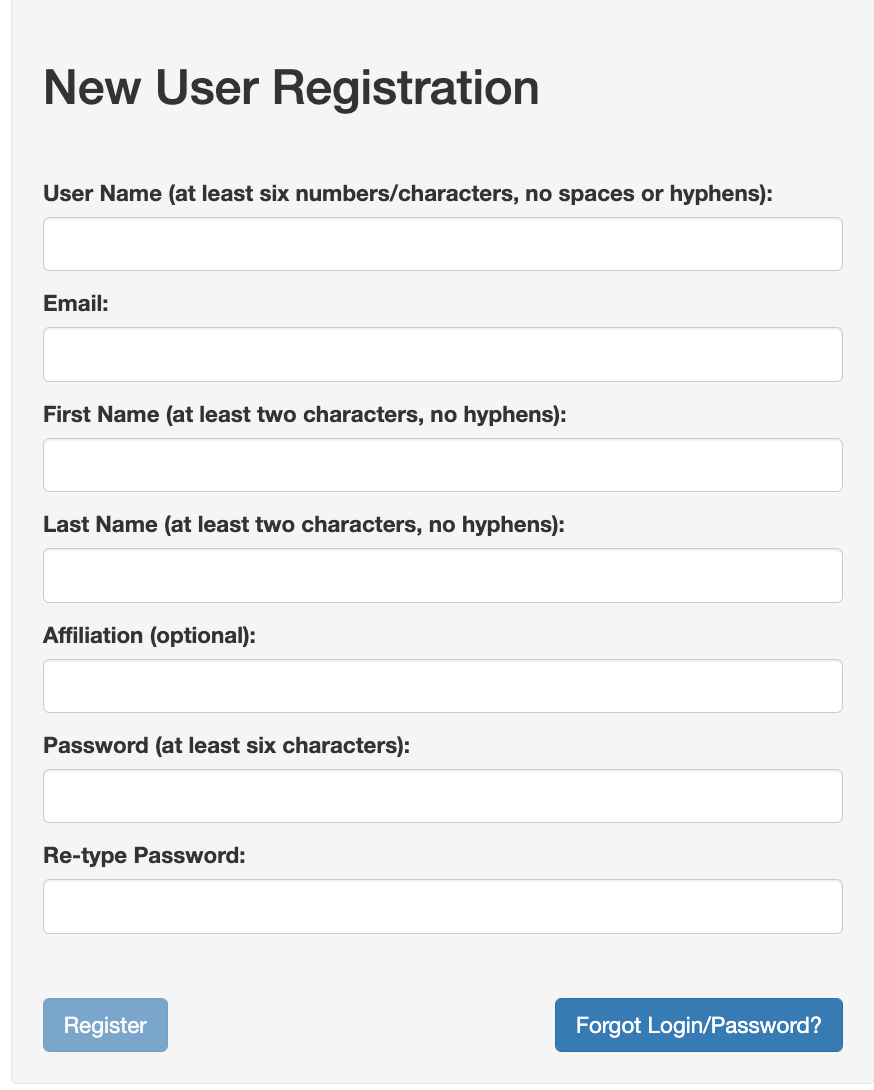
First time users must register to gain access to our system. Please use your email registered in the Group and Personnel Management System to register. One can access the Registration form by clicking the Register button shown in the login form - https://cspec.genome.network/cspec/ed/svi. That link will lead you to the form as shown below:
Requesting Access
- Log in to the Editor - https://cspec.genome.network/cspec/ed/svi once you are registered. The CSpec Editor ideally should match the vcep membership with GPM as soon as a registered user logs in. However, GPM integration to the CSpec and autoregistration feature will be available ONLY for the future releases of the Editor. First time users with no affiliation will see a message as shown below:
![noAffiliationInfo]()
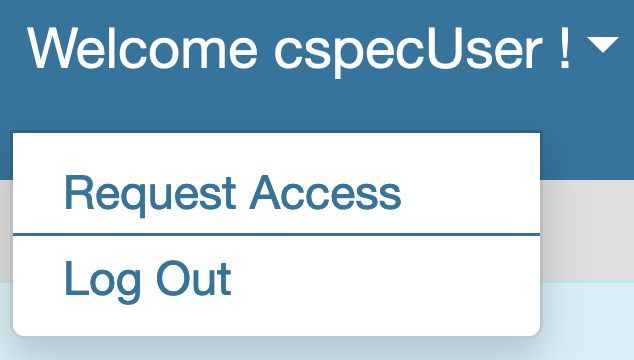
- Users can request access to a VCEP by using the Request Access feature at the top right hand side (below your account name) as shown below:
Request Access As a VCEP Coordinator/Member or SVI approver
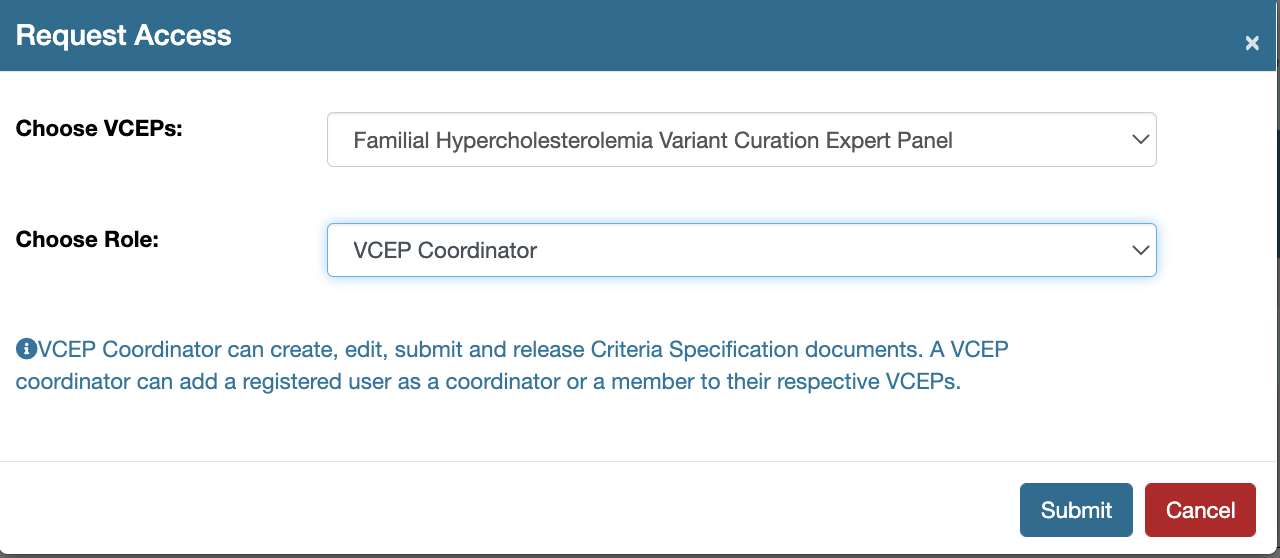
A user can request for a specific role (defined within the scope of CSpec Editor) to gain access to a specific VCEP.
- Choose the VCEP of interest
- Choose the Role from the three available options: a) VCEP Coordinator - VCEP Coordinator can create, edit, submit and release Criteria Specification documents. A VCEP coordinator can add a registered user as a coordinator or a member to their respective VCEPs. b) VCEP Member - VCEP member can create, edit Criteria Specification documents. c) SVI approver - Approves/reviews submitted specifications. Choose All VCEPs if you are requesting for this role.
Entry Page
Entry page shows a tabular view of all the specifications that a user has access to. The specification documents are grouped with respect to the VCEPs. Given below is an example of an entry page view for a test user who is a coordintor for multiple VCEPs. 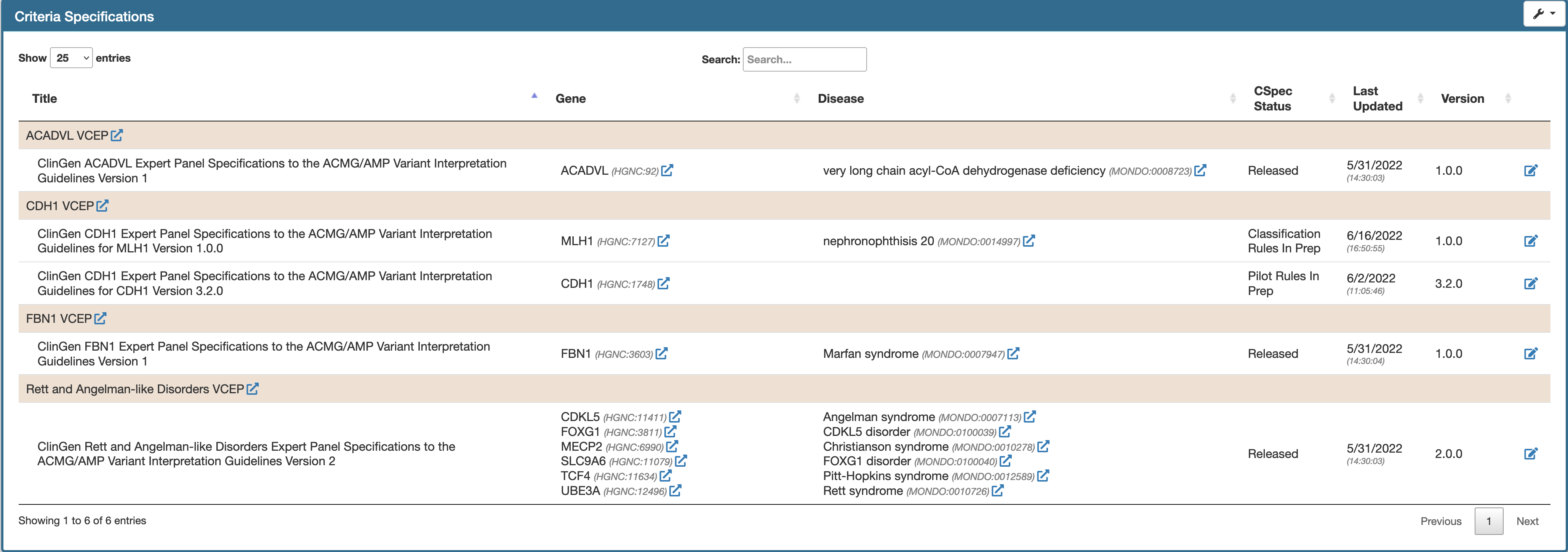 The table is:
The table is:
- By default sorted alphabetically by the VCEP name
- Each column is sortable individually.
- Pagination can be adjusted using the page controls on the top left hand side.
- Search field allows filtering specification document by gene, disease, status, etc.
Creating a new specification document
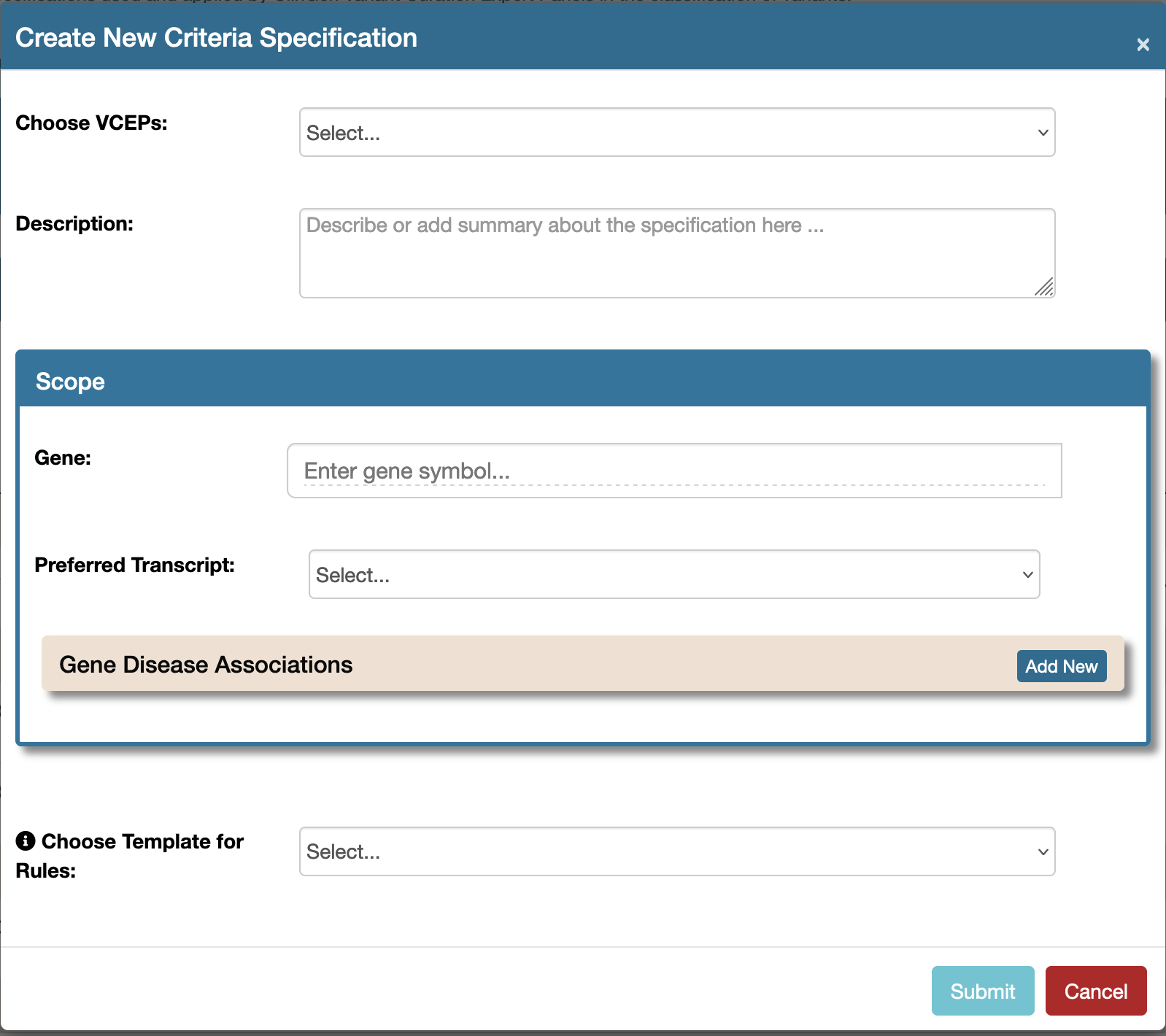
Click the Create New item in the Tools menu at the top right hand side of the Entry page as shown below:  Submit the create new form. The form Submit button will be disabled until all the required fields are selected or filled in:
Submit the create new form. The form Submit button will be disabled until all the required fields are selected or filled in:
Once a new document has been successfully created the success message will guide you to the new document like so:
Detailed view of a specification document
One can access the specification document page by clicking the pencil icon at the end of each row in the entry table. Sections of a specification document are:
General Metadata
Metadata about the specification include the title, version, current status, etc,.
Description
Description field of a specification document can be edited in either of the Prep stages - Classification Rules In Prep or Pilot Rules In Prep. Refer the illustrations below:

Please save the work once done:

A specification document goes through a series of stages, right from when a new document is created. These stage transitions are displayed at this section as breadcrumbs and an example is shown below. 
Changing the status of the document
One can change the status of the document using the tool option at the top right hand side and this feature is designed to respond based on the permissions a user has.  For instance a VCEP coordinator can submit the document in Classification Rules In Prep stage for approval (Classification Rules Submitted).
For instance a VCEP coordinator can submit the document in Classification Rules In Prep stage for approval (Classification Rules Submitted). 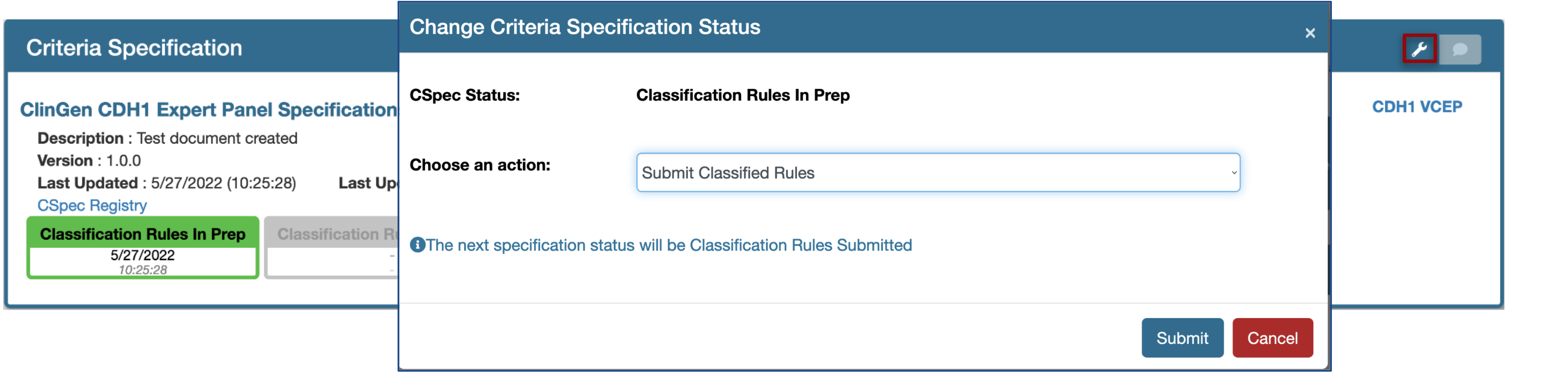 Once submitted, a coordinator can no longer make edits to that document. To make further edits the submission has to be either a) withdrawn - choosing the option Withdraw Classification Rules Submitted or b) must be formally approved by the SVI approver. Similarly, an SVI approver can approve OR review ( document returned without approval ) the submitted document to move the status of a document to the next stage, Pilot Rules In Prep or Classification Rules In Prep respectively.
Once submitted, a coordinator can no longer make edits to that document. To make further edits the submission has to be either a) withdrawn - choosing the option Withdraw Classification Rules Submitted or b) must be formally approved by the SVI approver. Similarly, an SVI approver can approve OR review ( document returned without approval ) the submitted document to move the status of a document to the next stage, Pilot Rules In Prep or Classification Rules In Prep respectively.
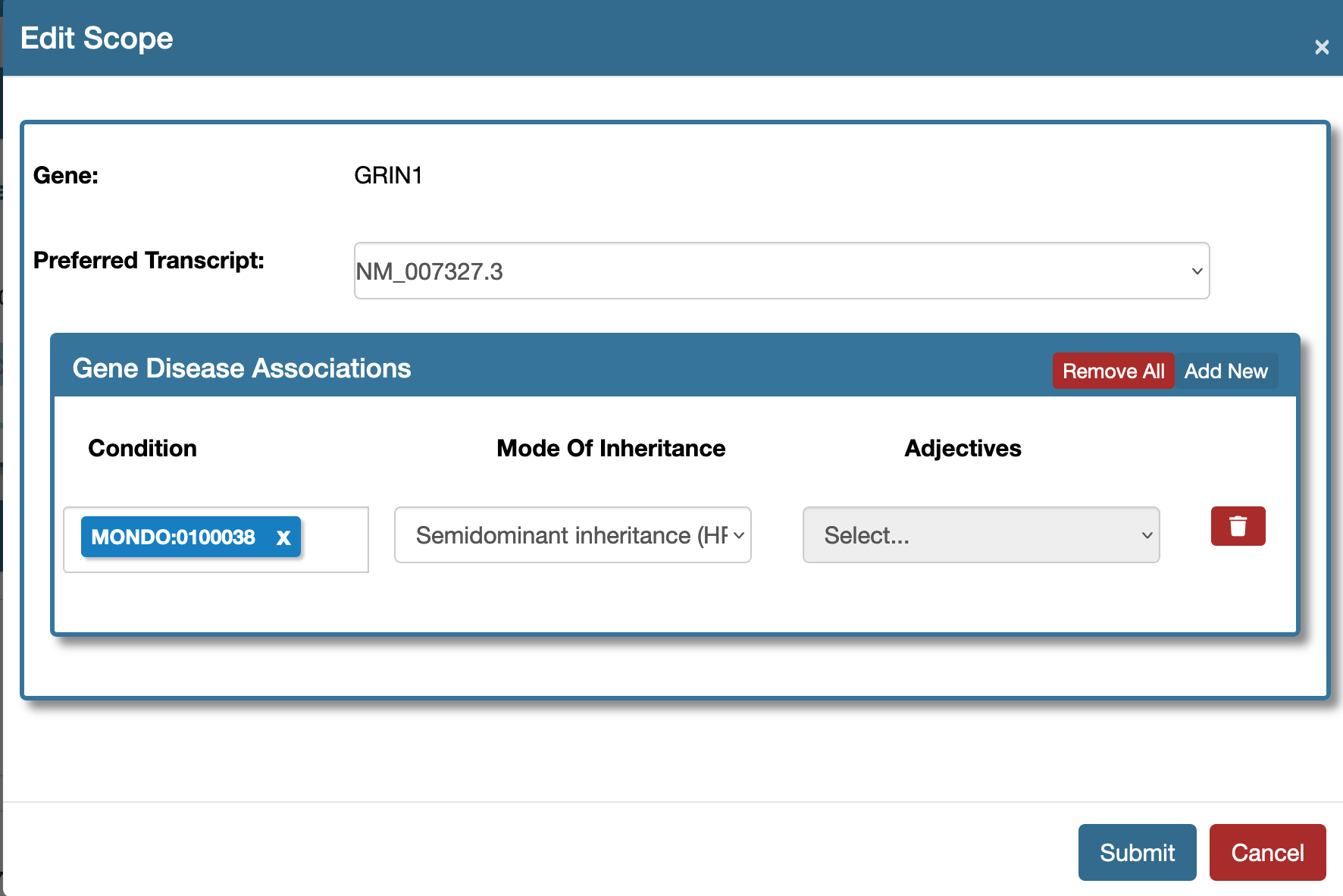
Change (update) the disease information
A VCEP coordinator can change the following properties which are defined as the scope of the document in either of the Prep stages (Classification Rules In Prep, Pilot Rules In Prep). Use the Edit Scope tool as shown here.
Fill the Edit Scope form and hit Submit to make the following changes:
- Transcript
- Add new diseases
- Remove previously existing diseases
- Update the mode of inheritance
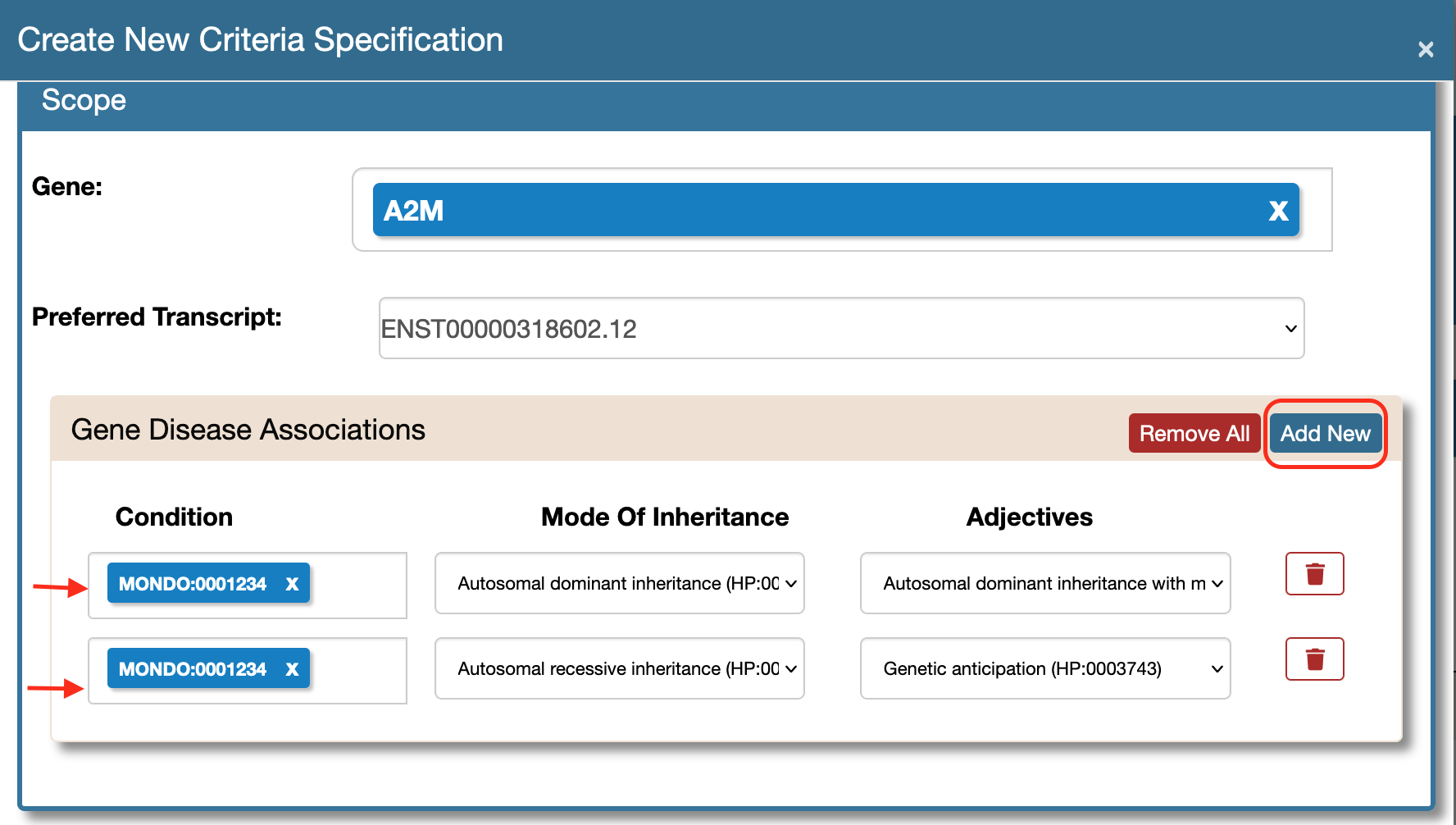
Adding multiple modes of inheritance to a disease entity.
Multiple modes of inheritance can be linked to a single disease entity either while creating a new document (using the Create New) or during any of the "Prep" stages (using the Edit Scope feature).
- Create New Specification Form And Multiple Modes Of Inheritance When creating a new document, you can associate multiple mois to a single disease. Access the Create New feature here:
Now at the Gene Disease Associations section use the Add New button to create multiple rows and select the same mondo ids. Choose different modes of inheritance and corresponding adjectives if any.
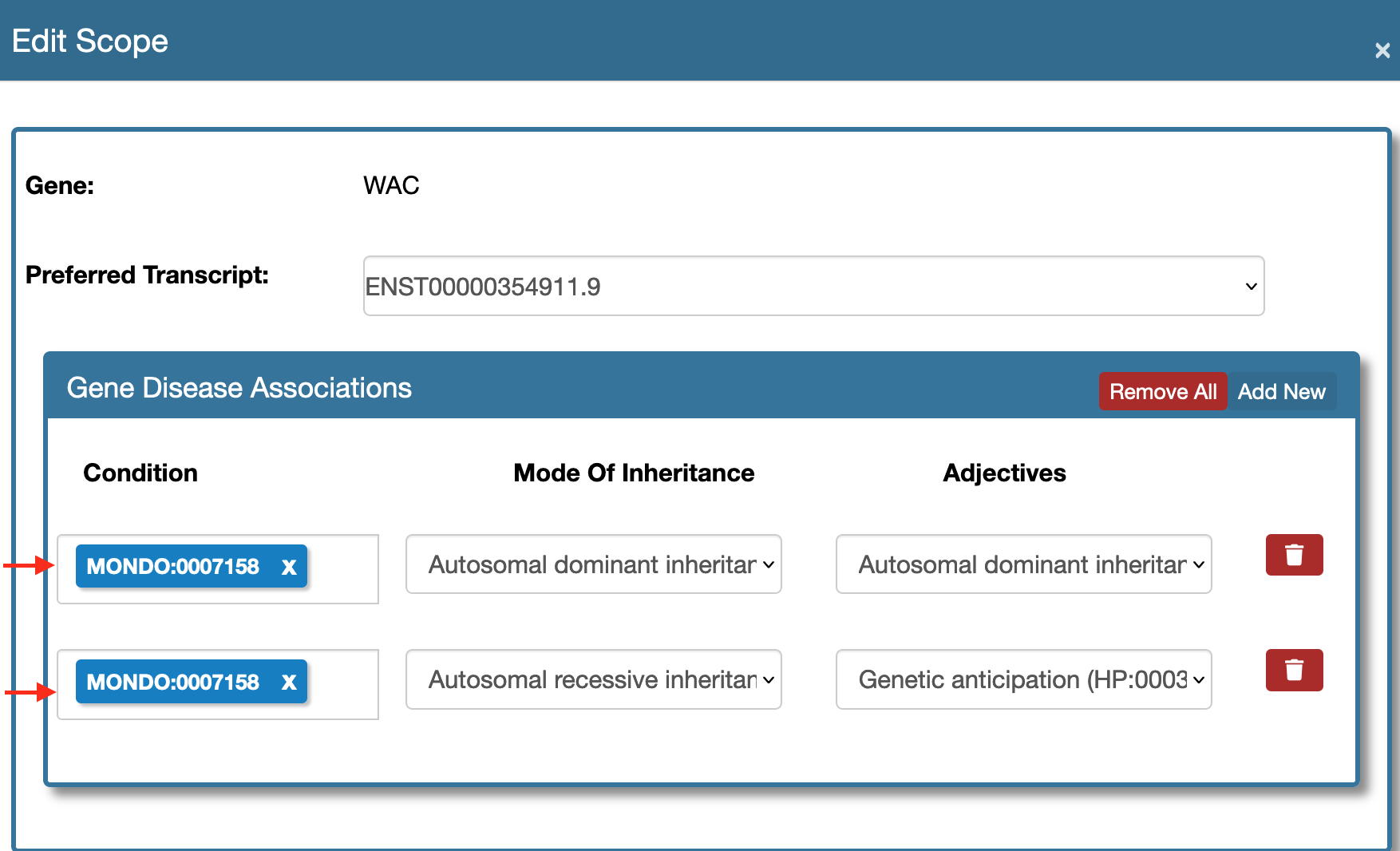
- Edit Scope Form And Multiple Modes Of Inheritance.
Use the Edit Scope tool as shown here.
Now at the Gene Disease Associations section use the Add New button to create multiple rows and select the same mondo ids. Choose different modes of inheritance and corresponding adjectives if any.
Criteria & Strength Specifications
A VCEP coordinator or a member can edit the Criteria & Strength Specifications table. Details can be found here
Reopen a previously released/existing specification document
A Released specification document must be reopened in order to make further edits. The document then must be versioned and submitted for approval. To reopen the document use the tool option on the top right hand side of the specification document. Once reopened the document status will be Pilot Rules In Prep 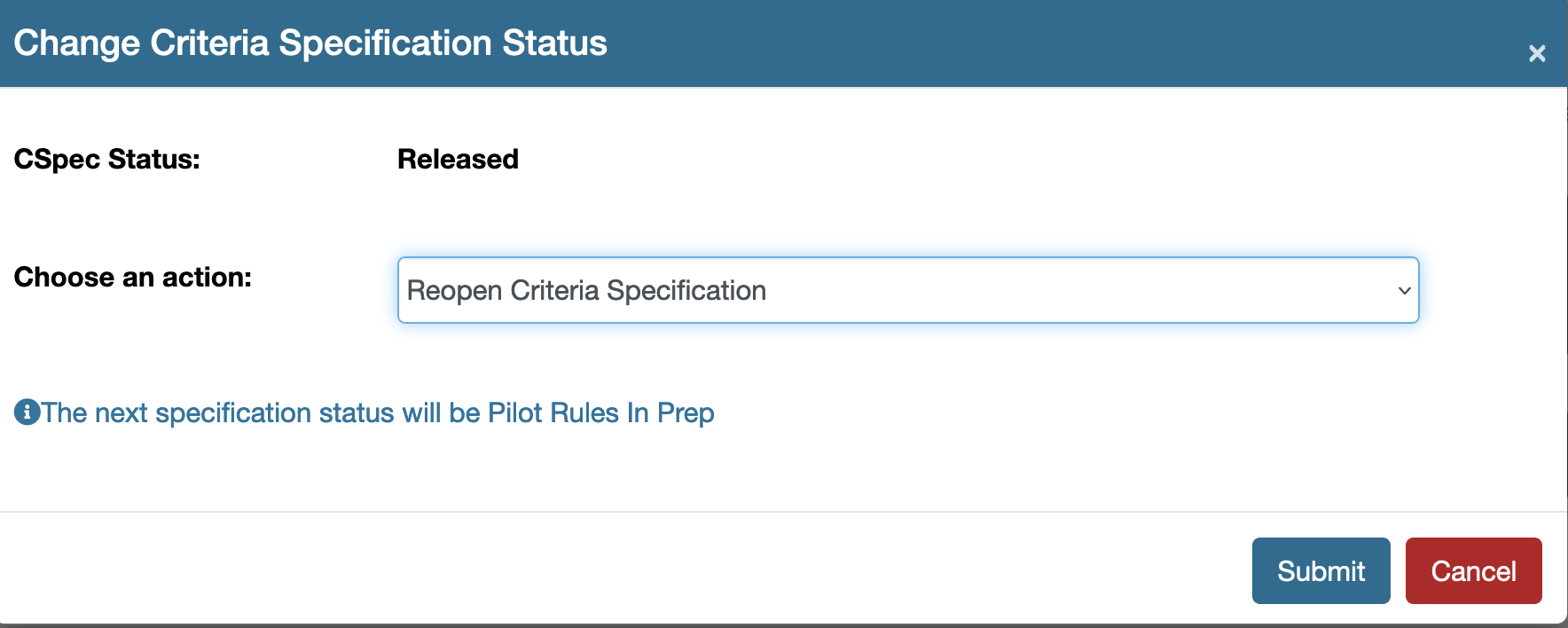
Re-submit the reopened document.
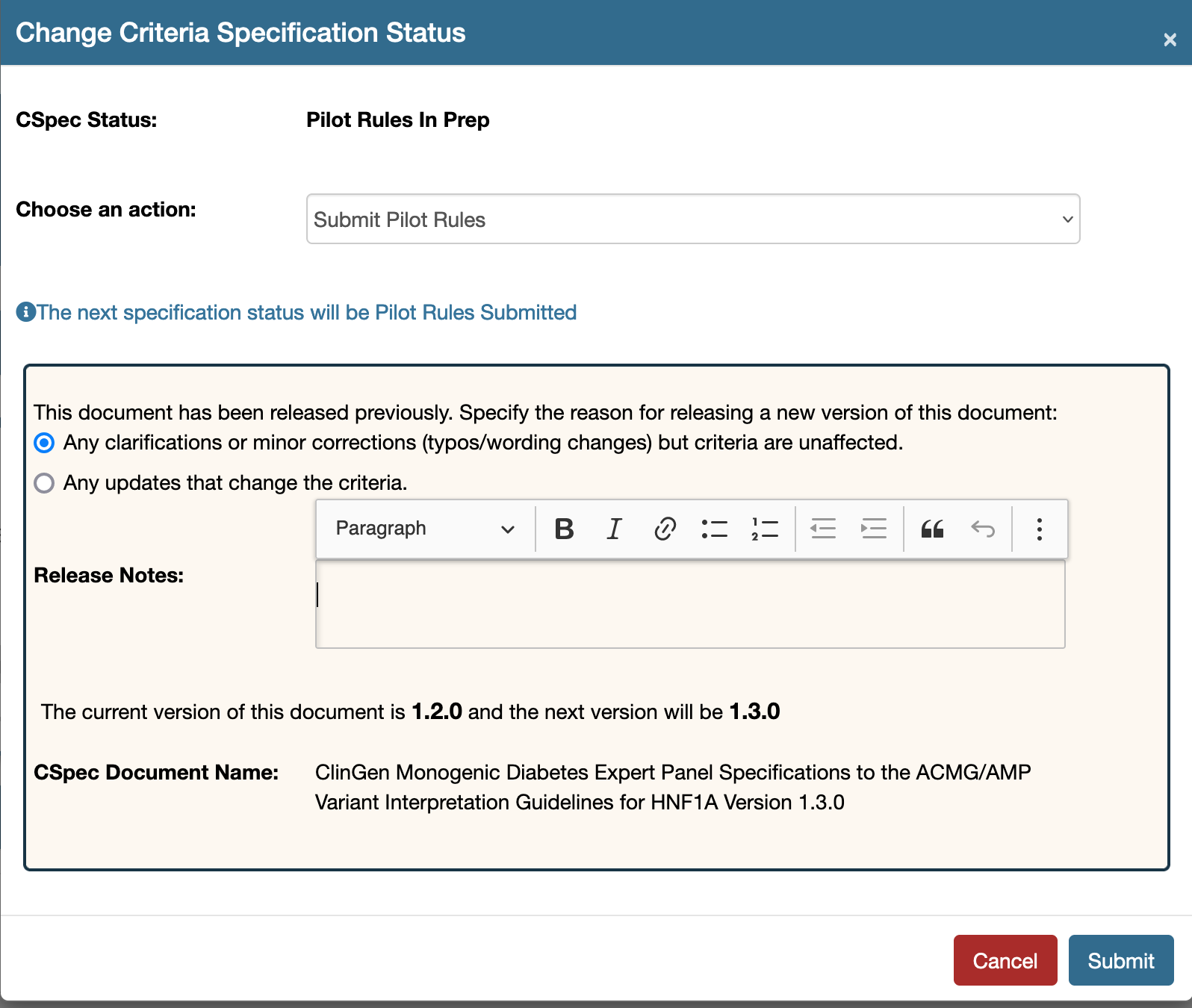
Once a reopened document has been edited, it must be submitted for approval by submitting the document. Click the tool option, as shown below at the top right hand side of the document to submit your document. 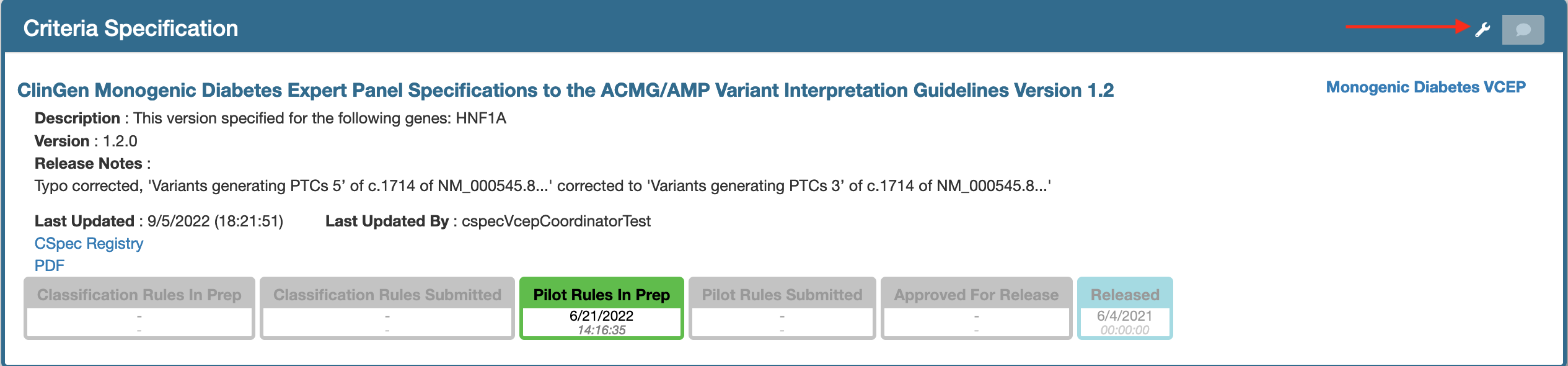
This tool will launch a modal as show below. Please mark the following:
- Correct version (minor or major with respect to the changes made)
- Release notes
- Click the submit button which will place the document in Pilot Rules Submitted
Change the version of a submitted document
Once submitted one can alter the version of the document by using the overwrite version feature. Withdraw the submission or if a submitted document is returned without approval this feature will be enabled. Use the tool option to see this feature. By default this is set to No. 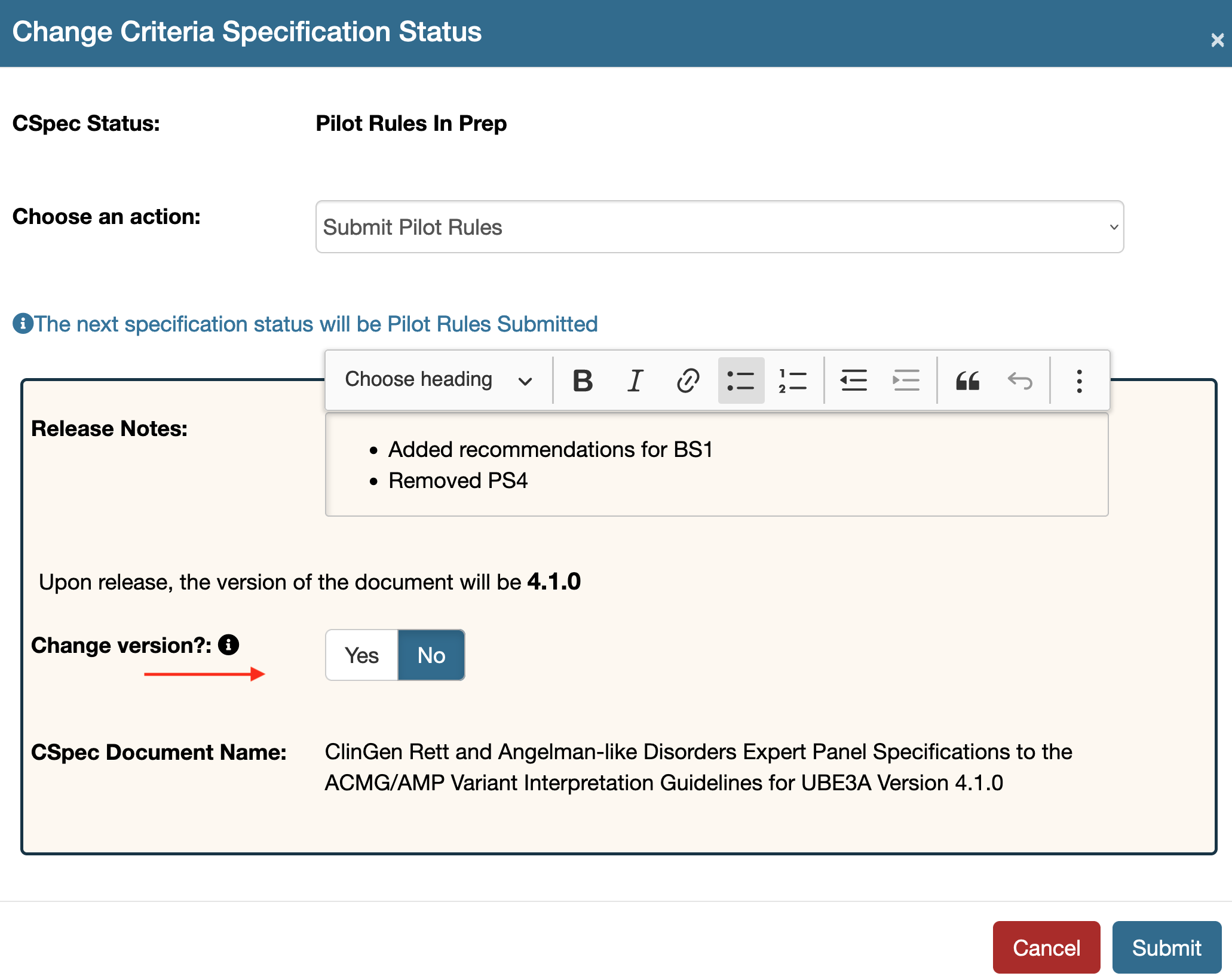 If the Yes button is clicked for the Change version? field an additional input field will appear. Fill in the new version here which will overwrite the running version of the document. Make sure to add all the three places in your version, that is it must be of the form - 1.0.0
If the Yes button is clicked for the Change version? field an additional input field will appear. Fill in the new version here which will overwrite the running version of the document. Make sure to add all the three places in your version, that is it must be of the form - 1.0.0  Submit your document once you have the correct version entered as shown in the example below:
Submit your document once you have the correct version entered as shown in the example below: 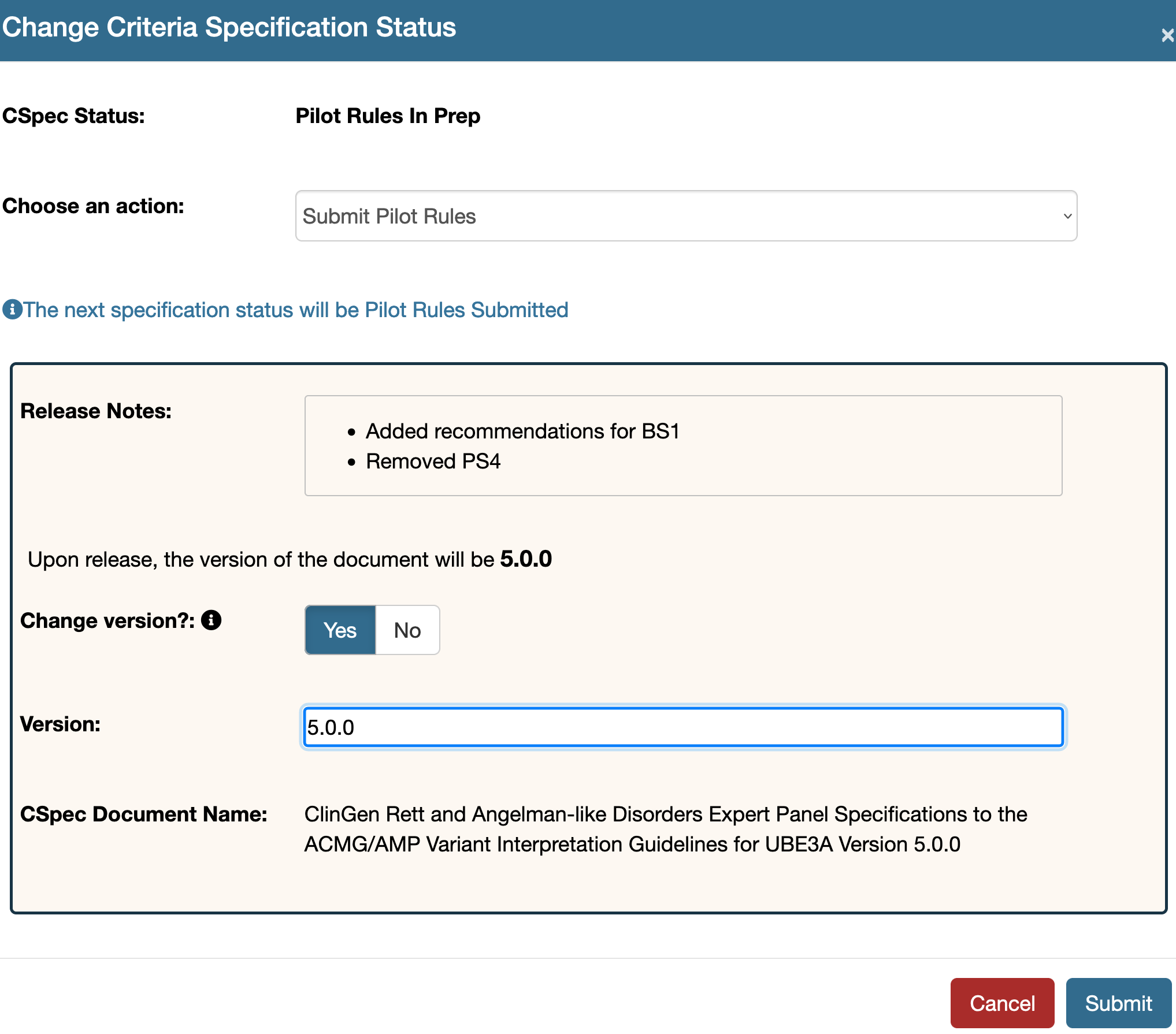
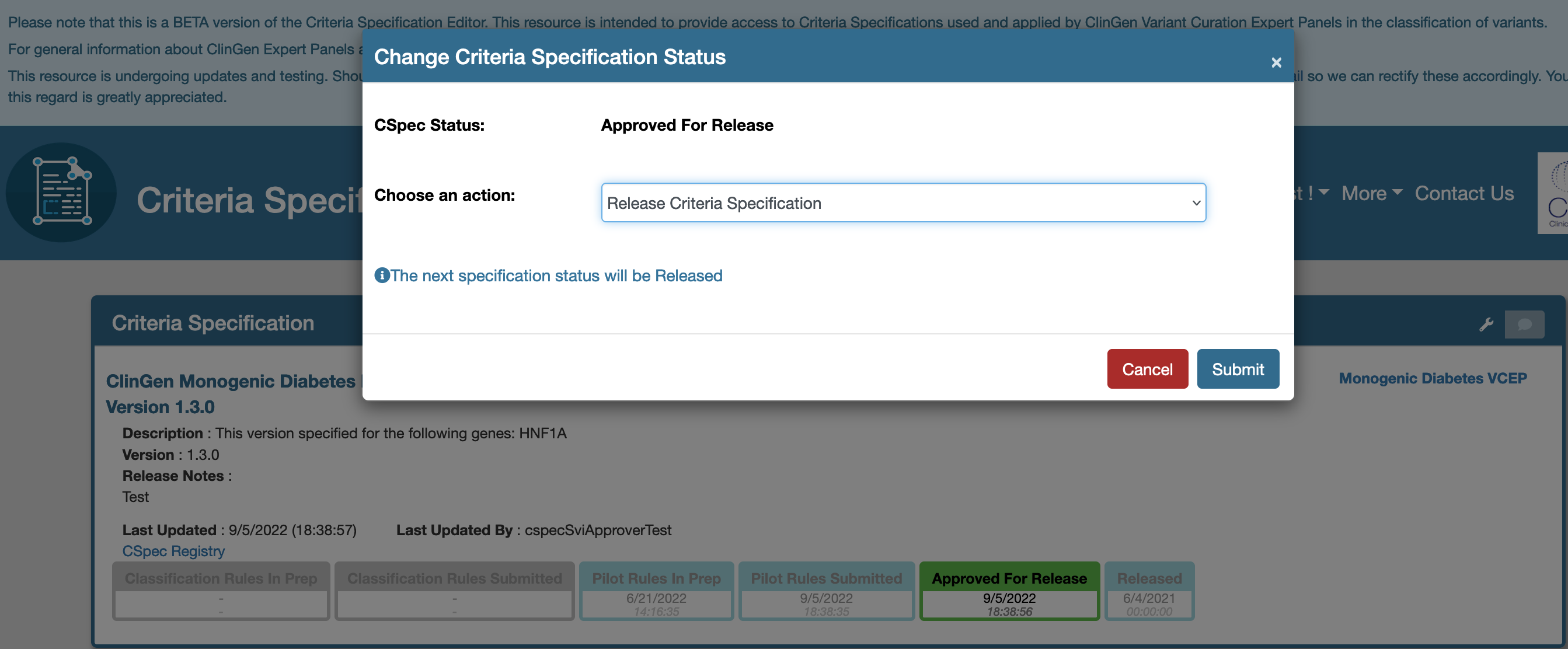
Release an approved document
Releasing a document will publish all the content of the approved document to
- The CSpec Registry
- The Data Exchange Kafka service, thereby making it available for VCI and other applications.
Follow the steps below to release a document. To release a document, the submitted document must be approved and placed in Approved For Release state as shown below. Review the content and make all the required changes before a document is released.
- Change the status of the document by clicking the top right hand side tool option.
- This will launch a release option and submit the form.
![release]()
Type-specify a criteria specification document
All the documents when newly created or prior to submission or release MUST be marked with a type. Follow the instructions here to specify your document type - https://genboree.org/gitlab/clingen/cspec/cspec-editor/-/wikis/Specification-types